Background:
Patients that have triple-class relapsed/refractory multiple myeloma (RRMM) have relapsed on each of the following classes: an immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), and anti-CD38 monoclonal antibody (MoAb). RRMM poses a significant challenge with poor prognosis due to limited options. We evaluated VDPACE (bortezomib, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide) for efficacy and safety as salvage therapy in heavily pre-treated RRMM.
Methods:
We retrospectively analyzed 54 patients who had triple-class RRMM and received at least 1 cycle of VDPACE between June 2016 - July 2023 at the University of Kansas Health System in collaboration with the U.S Myeloma Innovations Research Collaborative (USMIRC). VDPACE consisted of bortezomib 1 mg/m2 subcutaneous on days 1,4,8 and 11, doxorubicin 10 mg/m2 continuous infusion (CI) x 4 days, cisplatin 10 mg/m2 CI x 4 days, cyclophosphamide 400 mg/m2 CI x 4 days and etoposide 40 mg/m2 CI x 4 days. Descriptive analysis was performed on available data for patient characteristics, disease course, and outcomes differentiating these two groups. Adverse events were graded based on the CTCAE v5.0 criteria. Responses to therapy, including overall response rate (ORR), complete response or better (≥CR), and very good partial response (VGPR) were evaluated using the International Myeloma Working Group (IMWG) criteria. The Kaplan-Meier method was used for progression-free survival (PFS) and overall survival (OS) assessments.
Results:
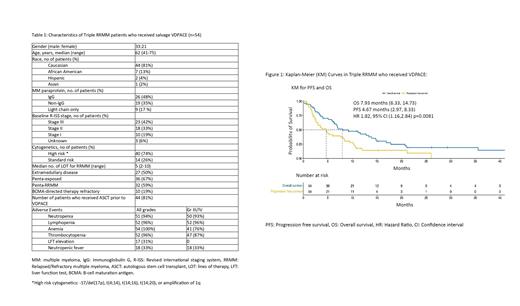
Of the 54 patients in this study, the median age was 62 years (41-75), 81% (n=44) were Caucasian, 48% (n=26) were IgG subtype, 42% of the patients (n=23) had Stage-III disease at treatment, 74% (n=40) had high-risk cytogenetics, and extramedullary disease (EMD) was present in 50% (n=27) of patients. These patients had a median of 5 (2-10) prior lines of therapy;67% (n=36) were penta-drug exposed, 59% (n=32) were penta-RRMM and 19% (n=10) were refractory to BCMA-directory therapy. Patient characteristics are summarized in Table 1. The overall response rate (ORR) for the whole cohort was 54%, 7 (13%) patients achieved ≥ VGPR, median PFS was 4.67 months (95% CI, 2.97 to 8.40), while OS was 7.93 months (95% CI, 6.33 to 14.73), see Figure 1. Regarding efficacy on penta-RRMM (n=32) vs non-penta-RRMM subgroups (n=22), the ORR was 44% vs 68% respectively, while the median PFS was 2.67 months (95% CI, 2-7.6) in the penta-RRMM subgroup compared to 9.28 months(95% CI, 4.67-NA) in the non-penta-refractory subgroup. Treatment post salvage therapy of VDPACE included autologous/allogenic stem cell transplant (SCT), BCMA-directed therapies (CAR-T, T-cell engaging bispecific antibodies and antibody-drug conjugates), other chemotherapies, in addition to enrollment to clinical trials reported at 13%, 26%, 39% and 4%, respectively. 11% patients proceeded to hospice due to progression disease.
Most patients experienced hematological adverse events (AEs), including grade III/IV lymphopenia (96%), neutropenia (93%), thrombocytopenia (87%) and anemia (76%). 69% (n=37) required a blood transfusion and 67% (n=36) required platelet transfusion. Neutropenic fever (NF) was reported in 33% (n=18), while the most common cause of infection was bacteremia in 24% of patients, followed by pneumonia in 20% of the patients. Following the initial hospitalization to receive treatment, the readmission rate within 60 days was 50% (n=27), that was mostly due to NF (52%, n=14). Treatment related mortality (TRM) in the first 30 days was reported in 6% (n=3), all due to severe sepsis. See Table 1.
Conclusions:
Based on our retrospective study, VDPACE is an effective salvage treatment in triple RRMM, and can be used as a bridging therapy prior to CAR-T, prior to enrollment in clinical trials or proceeding to SCT, however, due to TRM secondary to severe infections, careful selection of patients for this intensive therapy must be emphasized.
Disclosures
Mahmoudjafari:Genentech, Inc.: Consultancy; Pfizer, Genentech, Inc., BMS, KITE, Sanofi, Janssen: Honoraria; Omeros: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal